The Clinical Research Platform
The Principle
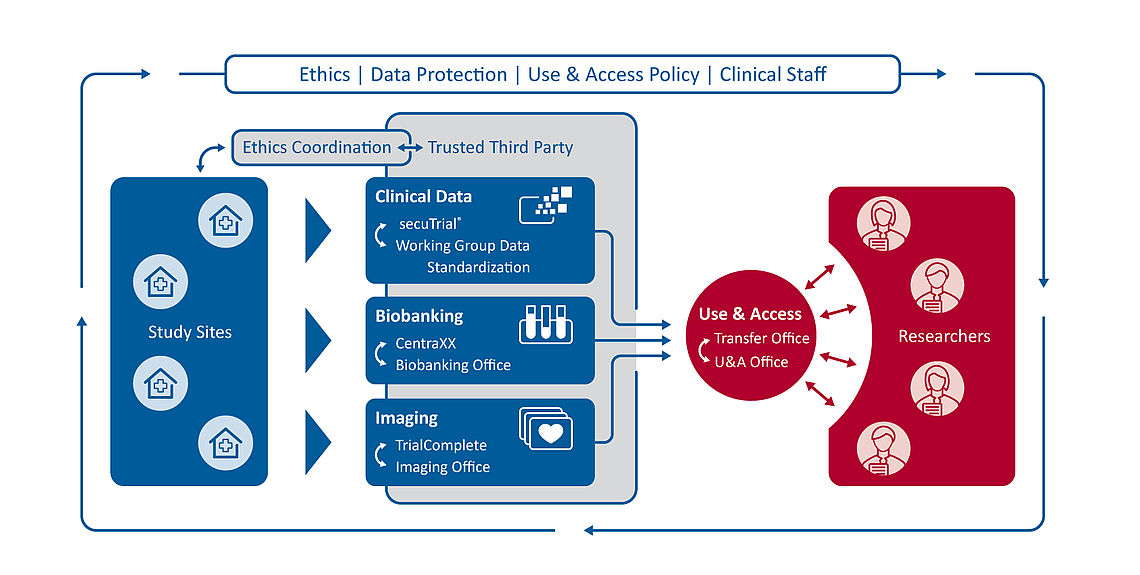
The clinical research platform consists of IT infrastructures, processes and rules that define the framework for all clinical studies at the DZHK (see graphic on the left). All studies financed by the DZHK use this platform. In this way, data and biosamples are recorded uniformly and are available for later research questions. About the research platform
The Quality
The DZHK has developed Standard Operating Procedures (SOPs). In clinical studies, these make it possible to generate and process data and biomaterials under standardized conditions. The clinical SOPs and the SOPs for DZHK biobanking are listed with the currently valid version on this website in the corresponding area
The Studies
Numerous DZHK studies include participants in the clinical research platform; around 130 national and international centers are involved. With our systems, we collect clinical data, image data and biosamples. All Studies
The Research
The DZHK makes the essential data and samples from the studies available to the worldwide scientific community within the framework of the DZHK Heart Bank for scientific questions. A usage regulation regulates this process. DZHK Heart Bank