Cooperation with Study Sponsor
Before you can use the technical infrastructure of the clinical research platform, you must conclude a study centre agreement with the study sponsor or the principal investigator. You must also obtain a local ethics vote from your ethics committee.
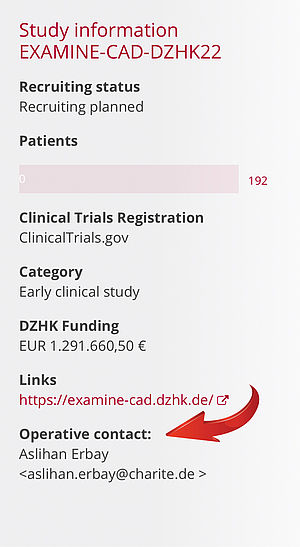
You can obtain all the documents and information you need for these processes from the principal investigator (PI). If you do not know the contact to your PI, you can look in our study overview on the DZHK Internet and find the operative contact there.
Here is an Example: